Alkaline Diets
The General Theory
The concept of balancing your bodies pH through diet has been around for a very long time (acid-base homeostasis or acid-base balance). Thirty years ago, I was using alkaline foods, such as wheatgrass, in my diet to help me recover from workouts for Ironman races. Today I am still using the same concepts to help me recover from fitness workouts, occasional injuries, and to keep my biological clock from rushing forward (I will explain that one later).The Alkaline Diet postulates that our bodies are designed for predominately alkaline foods, not the acidic diets of today (high meat, high grain, processed foods). There is considerable evidence to support the concept that acid-based diets are directly related to the degenerative, cardiovascular, and pathological conditions that are so prevalent today. Even inflammation, and weight gain have been associated with acidic diets.
Before we get into dietary details lets do a quick review of acidity and alkalinity.
Your Body's pH
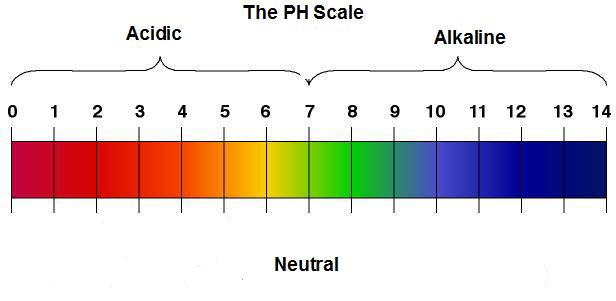
pH is a measure of the acidity or alkalinity of a solution. Solutions with a pH less than seven are acidic, while those with a pH greater than seven are basic (alkaline). A pH of 7 is neutral. The pH of your blood is maintained in very narrow range from 7.38 to 7.42. Even a minor deviation from this range can have severe consequences, sometimes even resulting in death. Your body maintains this narrow balance through three mechanisms: your respiratory system, chemical buffers, and your kidneys (excreting acid).
The balance between acids and bases in your body is of enormous importance to the functioning of all your metabolic processes. This balance is critical for the functioning of your proteins, the permeability of your membranes, the delivery of electrolytes, and the functioning of every cell, organ, and structure in your body.
- Your Respiratory System: When acidity increases there will be a corresponding increase in pulmonary respiration. This will cause an increase in the excretion of H+. Hydrogen molecules are what make a solution acidic. Then a secondary reaction occurs (H + HC03 = H2CO3) forms water (H2O) and carbon dioxide (CO2), which is then eliminated through respiration.
- Chemical Buffers - When your body buffers a solution, the buffer binds to the loose hydrogen. Removal of loose hydrogen from a solution makes it less acidic. These reactions can occur within seconds.
- Your Kidneys - The kidneys can balance blood pH by excreting excess acids (or bases). This is a much slower process than the respiratory mechanisms or the chemical buffers.
The balance between acids and bases in your body is of enormous importance to the functioning of all your metabolic processes. This balance is critical for the functioning of your proteins, the permeability of your membranes, the delivery of electrolytes, and the functioning of every cell, organ, and structure in your body.
Earlier, I mentioned that we can use the concept of alkaline diets to keep our biological clocks from rushing forward. Let me explain why.
As we age, the body's ability to remove acidity decreases (by up to 80% by the later part of your life). This is primarily due to decreased kidney function that occurs with normal aging (especially after age 50). This is due to a decrease in glomerular filtration. The important point here is that your acid-base balance is dependent upon your kidney's ability to remove acid from your body, and that this ability decreases with age.
This decreased kidney function can lead to a state known as low-grade metabolic acidosis (increase in H+). Low-grade metabolic acidosis is a clinical disorder that is characterized by an increase in total body acidity.Even mild acidosis increases bone loss, muscle wasting, and inflammation. Not what you want to do if you are trying to repair your body or increase your athletic performance. If we can avoid increased acidity in our bodies, we can slow down or avoid some of the diseases associated with aging and acidity.
As we age, the body's ability to remove acidity decreases (by up to 80% by the later part of your life). This is primarily due to decreased kidney function that occurs with normal aging (especially after age 50). This is due to a decrease in glomerular filtration. The important point here is that your acid-base balance is dependent upon your kidney's ability to remove acid from your body, and that this ability decreases with age.
This decreased kidney function can lead to a state known as low-grade metabolic acidosis (increase in H+). Low-grade metabolic acidosis is a clinical disorder that is characterized by an increase in total body acidity.
Diet and pH
The food you eat can have an effect on the how much stress is place on your body as it attempts to maintain its pH balance. Generally speaking, the North American diet is extremely acidic, because of the quantity of grains, animal products, and processed foods that is consumed. This constant acid-state does put a considerable load on your body's buffering systems.As we already mentioned the pH of your blood is kept within a very narrow pH range - around 7.4. Your body's buffering systems does this by drawing upon minerals from your bones (calcium, magnesium, potassium, zinc), amino acids from your muscles (glutamine), or by using your respiratory or kidney's buffering systems.
In
contrast to the pH of your blood, the pH of your urine varies based on your
diet. Most people in North America have a very acidic diets, which shows up
directly in their urinary pH. Even
consuming a high-salt diet can cause your body to create a state of low-grade
acidosis. Something to think about - that extra table salt you are adding to your food may be
leading to chronic conditions such as osteoporosis, slowing your healing, inflaming your body, and even making you gain weight.
Acidic states damage your body
As mentioned, most
North American diets produce a state of “chronic low grade acidosis”. What this
means is that our bodies are continuously pulling key minerals out of our bones, and amino acids from our muscles, in an attempt to maintain a constant pH in our blood and
cells.
Bone and muscle breakdown are two classic examples:
Bone Degeneration - Close
to a century ago, medical research concluded that constant acidic states in our
bodies cause the release (leaching) of calcium from our bones. This is because calcium is a strong base, used by the body to neutralize the acid. The net result
is osteoporosis, or weak, fragile, thin bones.
Muscle Break Down – Your muscles contain high levels of an amino acid called glutamine. This amino acid helps you to build and preserve muscle mass. Your body also uses this amino acid to balance your pH. When your body becomes too acidic, it will start to break down the glutamine in your body. This drives the pH scale to a more alkaline direction.
Side Note: Glutamine is definitely a supplement that I recommend taking on a regular basis to my patients. A decrease in glutamine levels leads to muscle wasting and suppressed immune function. Glutamine will:
Let me explain. We know that protein is essential for maintaining our lean muscle mass. The problem is that the high protein diet maintained by many athletes can cause an increase in acidosis, which can lead to the breakdown of both muscle and bone.
If we can decrease this acidity through diet and supplement, it is possible to avoid this from happening. For example, supplementation of sodium citrate and sodium bicarbonate has been shown to have substantial and positive effect on decreasing metabolic acidosis. Research has also shown that a more alkaline pH increases the removal of hydrogen ions from your muscles, which correlates to an increase in peak power output.
This really is worth doing. Within a few weeks, I believe you will be pleasantly surprised. The most common results I see within this time-period are:
This list helps you to determine, with reasonable accuracy, the effects of diet on the acidity and alkalinity in your body. This list can help you to lower your overall acidic load, remove a huge stress on your body's buffering systems, and help your body to healing itself.
In the PRAL list, most fruits and vegetables are alkalizing. Processed or refined foods are very acidifying. Meats are also acidifying, but will vary depending based on the type of meat being consumed. Most grains are also acidifying, but it is often surprising which ones cause the greatest increase of acidity. For example, brown rice is more acidic than white rice. Use the following list to balance out your diet. You will be surprised and pleased by the results you can achieve. Please note that many of the comments on this list are my own.
Side Note: Glutamine is definitely a supplement that I recommend taking on a regular basis to my patients. A decrease in glutamine levels leads to muscle wasting and suppressed immune function. Glutamine will:
- Help to maintain pH balance.
- Strengthen the immune system.
- Help promote weight loss through insulin control.
- Support gastrointestinal function.
- Help to build and preserve muscle mass.
- Help to control cravings for sugar and alcohol.
Athletic Performance
Balancing the pH of an athlete's diet will have two major effects:- It prevents hard-earned muscle from being broken down.
- It helps to increase their peak power output.
Let me explain. We know that protein is essential for maintaining our lean muscle mass. The problem is that the high protein diet maintained by many athletes can cause an increase in acidosis, which can lead to the breakdown of both muscle and bone.
If we can decrease this acidity through diet and supplement, it is possible to avoid this from happening. For example, supplementation of sodium citrate and sodium bicarbonate has been shown to have substantial and positive effect on decreasing metabolic acidosis. Research has also shown that a more alkaline pH increases the removal of hydrogen ions from your muscles, which correlates to an increase in peak power output.
Solutions
Balancing acid-base in your body does not not have to be daunting task. In the system that we recommend, you don't have to avoid acidic foods. However, it is important for you to balance acidic foods with alkaline foods. Instead of filling your plate with foods that are going to tip the pH scale to the acidic side, make sure that the majority of your choices are alkaline in nature.This really is worth doing. Within a few weeks, I believe you will be pleasantly surprised. The most common results I see within this time-period are:
- Decreased joint pain.
- Overall decrease in inflammation.
- Decreased blood pressure.
- Decreased headaches.
- Better sleep.
- Increase in mental clarity.
Foods To Eat, Foods to Avoid
The list that we have compiled is primarily based from the PRAL list. PRAL stands for Potential Renal Acid Load. The PRAL list was developed by two researchers - Remer and Manz.
This list helps you to determine, with reasonable accuracy, the effects of diet on the acidity and alkalinity in your body. This list can help you to lower your overall acidic load, remove a huge stress on your body's buffering systems, and help your body to healing itself.
In the PRAL list, most fruits and vegetables are alkalizing. Processed or refined foods are very acidifying. Meats are also acidifying, but will vary depending based on the type of meat being consumed. Most grains are also acidifying, but it is often surprising which ones cause the greatest increase of acidity. For example, brown rice is more acidic than white rice. Use the following list to balance out your diet. You will be surprised and pleased by the results you can achieve. Please note that many of the comments on this list are my own.
PRAL LIST:
PRAL List - 2004
IPEV Institute for Prevention and Nutrition, D-85737 Ismaning (copied with permission)
Food Table
The table allows for the assessment of dietary effects on acid-base balance.
Foods with a negative value
(blue colour) exert a base (B) effect (alkaline effect). These are the foods that you want to predominately eat and drink. The more negative the number in blue the better the health effect. These are the foods that you use to balance out the acidic foods (red colour).
The foods with a positive value have a acidic effect (A) effect. You don't have to avoid these foods, but you do need to balance them out with the foods/drinks in blue. Remember filling your plate up with just acidic foods is directly associated with causing degenerative/pathological conditions, inflammation, weight gain, and overall poor health.
Neutral foods are black in colour and labelled with the letter N.
Beverages
* Best Choices
** Worst Choices
Food
|
PRAL
|
|
Apple juice, unsweetened
|
B
|
-2,2
|
Beer, draft
|
B
|
-0,2
|
Beer, pale **
|
A
|
0,9
|
Beer, stout
|
B
|
-0,1
|
Beetroot juice
|
B
|
-3,9
|
Carrot juice *
|
B
|
-4,8
|
Coca-Cola **
Note: Diet Cola is even more acidic |
A
|
0,4
|
Cocoa, made with
semi-skimmed milk
|
B
|
-0,4
|
Coffee, infusion, 5
minutes
|
B
|
-1,4
|
Espresso
*
|
B
|
-2,3
|
Fruit tea, infusion
|
B
|
-0,3
|
Grape juice, unsweetened
|
B
|
-0,2
|
Green tea
, infusion *
|
B
|
-2,5
|
Herbal tea
|
B
|
-1,8
|
Lemon
juice
|
B
|
-0,1
|
Orange juice, unsweetened *
|
B
|
-2,9
|
Red wine
|
B
|
-2,4
|
Tea, Indian, infusion
|
B
|
-0,3
|
Tomato juice *
|
B
|
-2,8
|
Vegetable
juice (Tomato, beetroot, carrot) *
|
B
|
-3,6
|
White wine, dry
|
B
|
-1,2
|
Fats and Oils
* Best Choices
** Worst Choices
Food
|
PRAL
|
|
Butter
|
A
|
-2,2
|
Margarine
**
Note: Even though the margarine is basic, it is made from hydrogenated oils and cannot be recommended.
|
B
|
-0,2
|
Olive oil * - Olive oil is the oil of choice
|
N
|
0,9
|
Sunflower seed oil
|
N
|
-0,1
|
Nuts
Food
|
PRAL
|
|
Hazelnuts
|
B
|
-2,8
|
Peanuts, plain
|
A
|
8,3
|
Pistachio
|
A
|
8,5
|
Sweet almonds
|
A
|
4.3
|
Walnuts
|
A
|
6,8
|
Fish and Seafood
Note that the seafood with the highest level of acidity is shellfish. The key is not to avoid seafood, but the more acidic the seafood is, the more you will need to balance it with basic foods. For example a spinach salad.
Food
|
PRAL
|
|
Carp
|
A
|
7,9
|
Cod, fillets
|
A
|
7.1
|
Eal, smoked
|
A
|
11.0
|
Haddock
|
A
|
6,8
|
Halibut
|
A
|
7,8
|
Herring
|
A
|
7,0
|
Mussels
|
A
|
15.3
|
Prawn
|
A
|
15,5
|
Rose-fish
|
A
|
10,0
|
Salmon
|
A
|
9,4
|
Salted matie (herring)
|
A
|
8,0
|
Sardines in oil
|
A
|
13,5
|
Shrimps
|
A
|
7,6
|
Sole
|
A
|
7,4
|
Tiger Prawn
|
A
|
18,2
|
Trout, steamed
|
A
|
10,8
|
Zander
|
A
|
7,1
|
Fruits
Note how alkaline dried figs and raisins are. These are great alkaline foods to add to the mix.
Food
|
PRAL
|
|
Apples
|
B
|
-2,2
|
Apricots
|
B
|
-4,8
|
Bananas
|
B
|
-5.5
|
Black currants
|
B
|
-6,5
|
Cherries
|
B
|
-3,6
|
Figs, dried
|
B
|
-18,1
|
Grapefruit
|
B
|
-3,5
|
Grapes
|
B
|
-3.9
|
Kiwi fruit
|
B
|
-4.1
|
Lemon
|
B
|
-2,6
|
Mango
|
B
|
-3.3
|
Orange
|
B
|
-2,7
|
Peach
|
B
|
-2.4
|
Pear
|
B
|
-2,9
|
Pineapple
|
B
|
-2,7
|
Raisins
|
B
|
-21.0
|
Strawberries
|
B
|
-2,2
|
Watermelon
|
B
|
-1,9
|
Cereals and Flour
There are several factors to consider with grains, besides their acidity. This includes food allergies (wheat is a common allergy), nutrient value, and glycemic index.
Food
|
PRAL
|
|
Amaranth
|
A
|
7,5
|
Barley (wholemeal)
|
A
|
5,0
|
Buckwheat (whole grain)
|
A
|
3,7
|
Corn (whole grain)
|
A
|
3,8
|
Cornflakes
|
A
|
6,0
|
Dried unripe spelt grains (wholemeal)
|
A
|
8.8
|
Dried unripe spelt grains (wholemeal)
|
A
|
8,8
|
Millet (whole grain)
|
A
|
8,6
|
Oat flakes
|
A
|
10,7
|
Rice, brown
|
A
|
12,5
|
Rice, white
|
A
|
4,6
|
Rice, white, boiled
|
A
|
1,7
|
Rye flour
|
A
|
4,4
|
Rye flour, wholemeal
|
A
|
5,9
|
Wheat flour, white
|
A
|
6,9
|
Wheat
flour, wholemeal
|
A
|
8,2
|
Pasta
What is interesting about pasta is that, the more its cooked, the more acidic it becomes, and the higher it is on the glycemic index. If you are looking to balance out the acidity of meat, then a potato may be a better choice.
Food
|
PRAL
|
|
Macaroni
|
A
|
6,1
|
Noodles
|
A
|
6,4
|
Spaetzle (German sort of pasta)
|
A
|
9,4
|
Spaghetti, white
|
A
|
6,5
|
Spaghetti, wholemeal
|
A
|
7,3
|
Bread
Again acidity is not the only factor to consider. Are you allergic to wheat or have a sensitivity. If not, try to balance your bread intake with fruits, vegetables, and non-acidic drinks.
Food
|
PRAL
|
|
Bread, rye flour
|
A
|
4,1
|
Bread, rye flour, mixed
|
A
|
4,0
|
Bread, wheat flour, mixed
|
A
|
3,8
|
Bread, wheat flour, whole meal
|
A
|
1,8
|
Bread, white wheat
|
A
|
3,7
|
Coarse wholemeal bread
|
A
|
5,3
|
Crispbread, rye
|
A
|
3,3
|
Pumpernickel
|
A
|
4,2
|
Rusk
|
A
|
5,9
|
Wholemeal bread
|
A
|
7.2
|
Meat & Sausages
I am definitely a carnivore, but it really important to balance out the acidity of meat with alkaline foods.
Food
|
PRAL
|
|
Beef, lean only
|
A
|
7,8
|
Cervelat sausage
|
A
|
8,9
|
Chasseur sausage
|
A
|
7,2
|
Chicken, meat only
|
A
|
8,7
|
Corned beef, canned
|
A
|
13,2
|
Duck
|
A
|
4,1
|
Duck, lean only
|
A
|
8,4
|
Frankfurters
|
A
|
6,7
|
Goose, lean only
|
A
|
13,0
|
Lamb, lean only
|
A
|
7,6
|
Liver (veal)
|
A
|
14,2
|
Liver sausage
|
A
|
10,6
|
Luncheon meat, canned
|
A
|
10,2
|
Ox liver
|
A
|
15,4
|
Pig's Liver
|
A
|
15,7
|
Pork sausage
|
A
|
7,0
|
Pork sausage (Wiener)
|
A
|
7,7
|
Pork, lean only
|
A
|
7,9
|
Rabbit, lean only
|
A
|
19,0
|
Rump steak, lean and fat
|
A
|
8,8
|
Salami
|
A
|
11,6
|
Slicing sausage containing ham
|
A
|
8,3
|
Turkey, meat only
|
A
|
9,9
|
Veal, fillet
|
A
|
9,0
|
Milk, Dairy Products & Eggs
Eggs are great sources of protein, but considering the acidity of egg yolks, you are better off mixing one whole egg with extra egg whites. Check out the acidity of parmesan cheese, it tastes great, but is not the best food if you are trying to lose weight
Food
|
PRAL
|
|
Buttermilk
|
A
|
0,5
|
Camembert
|
A
|
14,6
|
Cheddar-type, reduced fat
|
A
|
26,4
|
Cottage cheese, plain
|
A
|
8,7
|
Cream, fresh, sour
|
A
|
1,2
|
Curd cheese
|
A
|
0,9
|
Edam Cheese full fat
|
A
|
19,4
|
Egg, chicken, whole
|
A
|
8,2
|
Egg, white
|
A
|
1,1
|
Egg, yolk
|
A
|
23,4
|
Emmental Cheese full fat
|
A
|
21,1
|
Fresh cheese (Quark)
|
A
|
11,1
|
Full-fat soft cheese
|
A
|
4,3
|
Gouda
|
A
|
18,6
|
Hard cheese
|
A
|
19,2
|
Kefir Cheese full fat
|
N
|
0,0
|
Milk, whole, evaporated
|
A
|
1,1
|
Milk, whole, pasteurised and sterilized
|
A
|
0,7
|
Parmesan
|
A
|
34,2
|
Processed cheese, plain
|
A
|
28,7
|
Rich creamy full fat cheese
|
A
|
13,2
|
Skimmed Milk
|
A
|
0,7
|
Whey
|
B
|
-1,6
|
Yogurt, whole milk, fruit
|
A
|
1,2
|
Yogurt,
whole milk, plain
|
A
|
1,5
|
Vegetables
This is the area that most people lack in their diet. Use these foods liberally to balance out your acidic foods.
Food
|
PRAL
|
|
Asparagus
|
B
|
-0,4
|
Broccoli, green
|
B
|
-1,2
|
Brussel sprouts
|
B
|
-4,5
|
Carrots
|
B
|
-4,9
|
Cauliflower
|
B
|
-4,0
|
Celery
|
B
|
-5,2
|
Chicory
|
B
|
-2,0
|
Cucumber
|
B
|
-0,8
|
Eggplant
|
B
|
-3,4
|
Fennel
|
B
|
-7,9
|
Garlic
|
B
|
-1,7
|
Gherkin, pickled
|
B
|
-1,6
|
Kale
|
B
|
-7,8
|
Kohlrabi
|
B
|
-5,5
|
Lamb's lettuce
|
B
|
-5,0
|
Leeks
|
B
|
-1,8
|
Lettuce
|
B
|
-2,5
|
Lettuce, iceberg
|
B
|
-1,6
|
Mushrooms, common
|
B
|
-1,4
|
Onions
|
B
|
-1,5
|
Peppers, Capsicum, green
|
B
|
-1,4
|
Potatoes
|
B
|
-4,0
|
Radish, red
|
B
|
-3,7
|
Ruccola
|
B
|
-7,5
|
Sauerkraut
|
B
|
-3,0
|
Soy beans
|
B
|
-3,4
|
Soy milk
|
B
|
-0,8
|
Spinach
|
B
|
-14,0
|
Tofu
|
B
|
-0,8
|
Tomato
|
B
|
-3,1
|
Zucchini
|
B
|
-4,6
|
References:
- Acid-base Metabolism. Nutrition, Health, Disease. European Journal of Nutrition Special Issue, Eur J Nutr 40:187-259 (2001)
- Acid-alkaline balance and its effect on bone health.Brown SE, Jaffe R, International Journal of Integrative Medicine 6:7-1 (2000)
- The effects of acid on bone. Bushinsky DA, Frick KK, Curr Opin Nephrol Hypertens 9:369-379 (2000)
- Remer T. Influence of Diet on Acid-Base Balance. Seminars in Dialysis. 2000; 13(4):221-226.
- Bone loss because of high sodium intake: Is there a connection to the acid-base balance? PETRA FRINGS, NATALIE BAECKER, ANDREA BOESE, MARTINA HEER Institute of Aerospace Medicine, DLR, Linder Hoehe, 51147 Cologne, Germany, 2nd International Acid-Base Symposium 2006
- Frassetto L, Morris RC, Sebastion A. Potassium Bicarbonate Reduces Urinary Nitrogen Excretion in Postmenopausal Women. J Clin Endocrinol & Metab. 1997; 82(1):254-259.
- Requena B, et al. Sodium Bicarbonate and Sodium Citrate: Ergogenic Aids? J Strength Cond Res.2005; 19(1):213-224
- Manz F, et al. Effects of a high protein intake on renal acid excretion in bodybuilders. Z Ernahrungswiss. 1995; 34(1):10-15.
- Potential renal acid load (PRAL) of foods and its influence on urine pH.Remer T, Manz F, Am Diet Assoc 95:791-797 (1995)
In Part 5 of "Dr. Abelson’s– Essential Nutritional Program", we will talk about weight loss.
To purchase our internationally best-selling books, visit www.releaseyourbody.com.
For information about our clinic in Calgary, Alberta, please go to www.kinetichealth.ca.
(COPYRIGHT KINETIC HEALTH 2013 – ALL RIGHTS RESERVED)



You know your projects stand out of the herd. There is something special about them. It seems to me all of them are really brilliant!
ReplyDeleteNutrition Programs Calgary
Hi I just started my blog and published my first post. These are really awesome tips. Thanks!
ReplyDeleteNutrition Programs Calgary